The species H2O2, ONOO⁻, NO, NO2⁻ being directly oxidized at their
€ 21.50 · 4.7 (393) · Auf Lager

Download scientific diagram | The species H2O2, ONOO⁻, NO, NO2⁻ being directly oxidized at their distinct potentials and their reconstructed fluxes. Reproduced from [2] with permission from Wiley from publication: Nanomaterial-based electrochemical sensors and optical probes for detection and imaging of peroxynitrite: a review | Peroxynitrite (PON for short) is a powerful nitrating, nitrosating and oxidative agent for cellular constituents. In vivo, PON is formed through the diffusion-controlled reaction between superoxide radical (O2•-) and nitric oxide (•NO). This critical review (with 67 refs.) | Electrochemical Sensors, Theranostics and Biocompatibility | ResearchGate, the professional network for scientists.

SOLVED: Consider the following reaction: 2NO3 (aq) + 4H2O (aq) +

Biochem Exam 2 Lecture 1 Flashcards

NO Oxidation Using H2O2 at a Single-Atom Iron Catalyst

Oxidative stress and antioxidants

Non-radical pathway dominated catalytic oxidation of As(III) with

H2O2 acts only as an oxidising agent. H2O2⟶H2O + O

Antioxidants, Free Full-Text
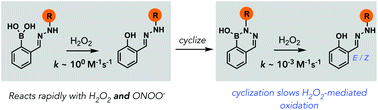
Diazaborines oxidize slowly with H2O2 but rapidly with
Solved The reaction mechanism proposed for the decomposition

Targeting the NO/superoxide ratio in adipose tissue: relevance to

Evaluation of a novel pyridinium cation-linked styryl-based

Reactions and transformations of the superoxide anion. SOD enzymes

Hydrogen Peroxide (H2O2)- and Nitric Oxide (NO)-Derived

/images/60090/600906239.jpg)