FDA Repeatedly Rejected Safety Claims About Philips Breathing Machines, Emails Show — ProPublica
€ 26.00 · 4.6 (496) · Auf Lager

As Philips reassured patients that millions of recalled machines were safe, internal emails show federal regulators privately told the company its testing didn’t account for the impact of long-term harm from tainted devices.
ProPublica New York NY
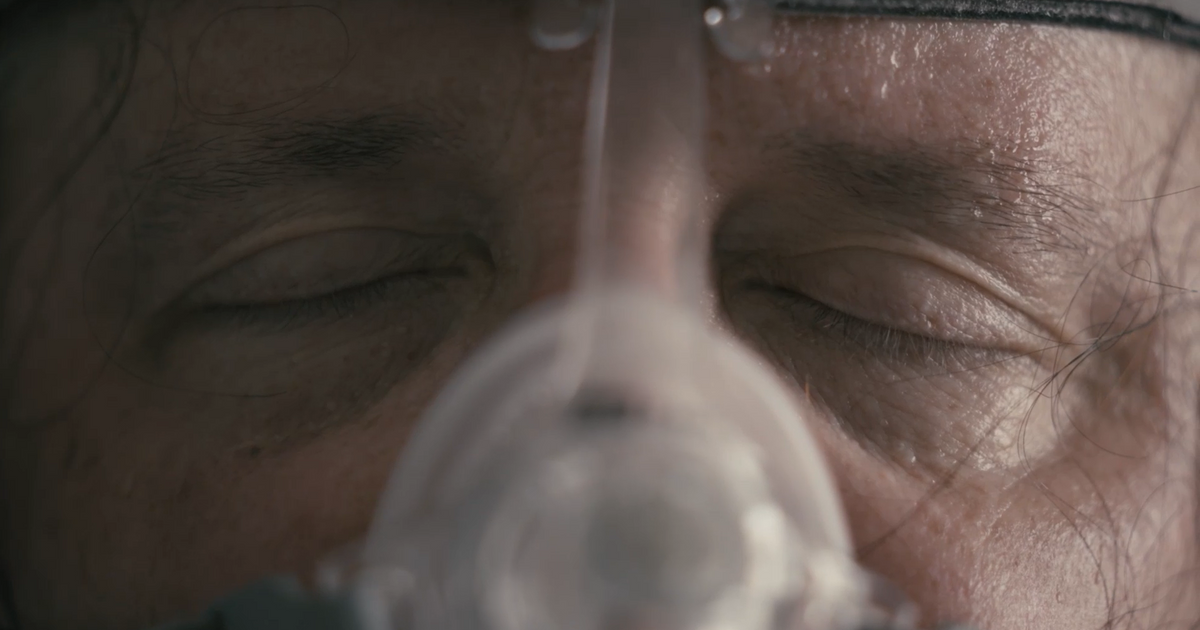
Philips Kept Complaints About Dangerous CPAP Machines Secret While Company Profits Soared — ProPublica

CPAP & PAP Devices

Philips Kept Complaints About Dangerous Breathing Machines Secret While Company Profits Soared
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/G76KUAQ7IFBB5MVGWJ3G6QQIEM.jpg)
FDA: Recalled Philips BiPAP, CPAP machines tied to more than 560 deaths – WPXI

FDA Repeatedly Rejected Safety Claims Made by Philips After the CPAP Recall but Waited to Alert the Public, Emails Show
Philips CPAP Recall Support Group

Brian Pierce MD (@brianpierce@) - Med-Mastodon
Pittsburgh Post-Gazette on LinkedIn: This vending machine, which features an advertisement for the 1999 film…
Philips CPAP Recall Support Group, Weekend update

Questions abound over future of Philips Respironics, a mainstay of Western Pennsylvania's economy

The BFD They Hid the Truth About Millions of Breathing Machines

Philips kept warnings about dangerous CPAP machines secret while profits soared

FOIA // FEED (@FOIAFeed) / X
ProPublica on X: .@ProPublica & @PittsburghPG found that the FDA received 1000s of complaints about tainted breathing machines ahead of the Philips CPAP recall but didn't alert doctors or patients. Now, senators